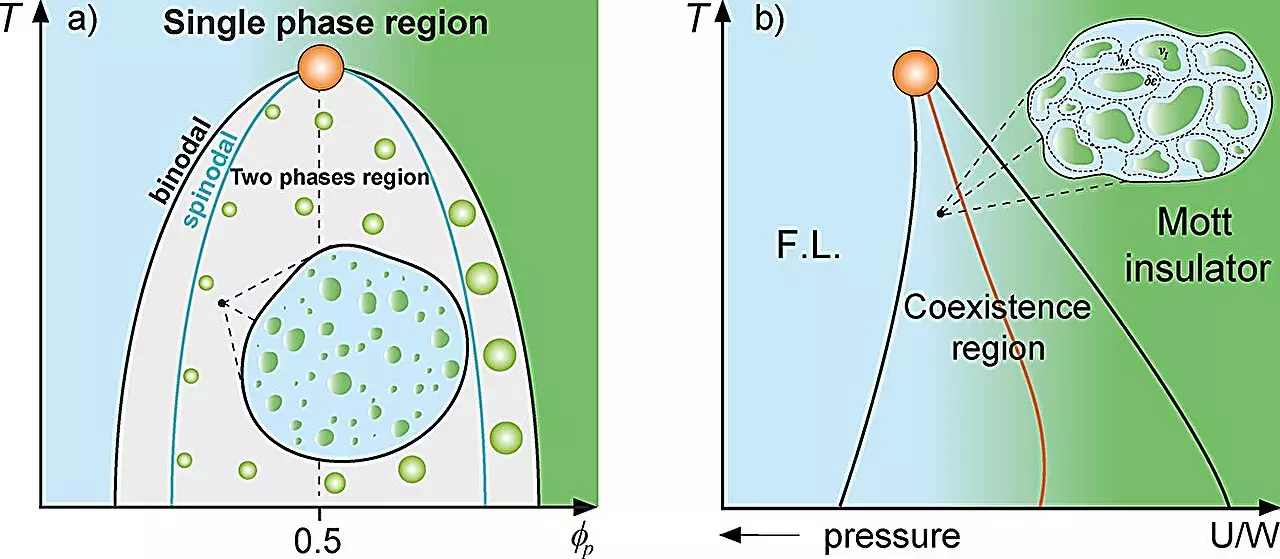
In the realm of physics, the understanding of complex systems often hinges on the principles of mixture theory. This framework assists scientists in modeling the interactions between multiple substances while accounting for their respective proportions. The study of supercooled water, for example, illustrates how different densities coexist within the same system, contributing to the formation of distinct phases. Another salient example is the Mott metal-insulator transition, where metal droplets dispersed in an insulating matrix exhibit unique physical behaviors. These principles of mixture theory have inspired recent interdisciplinary voyages into cellular biology, spearheaded by researchers at São Paulo State University (UNESP) in Brazil.
Notably, the work being conducted at UNESP focuses on the fascinating and complex phenomenon of protein compartmentalization in cells. The team, under the guidance of Mariano de Souza, has proposed a novel framework that draws parallels between classical Griffiths phases in magnetic systems and cellular environments. In traditional magnetic Griffiths phases, regions of magnetization can spontaneously arise in matrices that are either magnetized or non-magnetized. This spontaneous emergence leads to a significant slowdown in system dynamics. The researchers have adapted this theory to explain how similar “rare regions” can form in cellular contexts, providing a fresh lens through which to view protein behavior within biological systems.
The first author of the study, Lucas Squillante, elaborates on this innovative perspective, indicating that just as magnetized regions emerge in certain magnetic systems, protein droplets in cells can form under specific conditions, leading to reduced dynamics and a richer understanding of cellular functions.
Intriguingly, the formation of liquid-liquid phase separation in biological systems resonates powerfully with the principles of thermodynamics. As highlighted in the study, when protein concentrations within cells exceed a certain threshold, it triggers the creation of protein droplets—an essential mechanism for cellular organization and function. The researchers utilized established thermodynamic models, such as the Grüneisen parameter and the Avramov-Casalini model, to demonstrate that cellular dynamics are notably dampened near the critical binodal line of phase separation.
This understanding introduces a Griffiths-like cellular phase, which potentially sheds light on the origins of life. Drawing on historical theories proposed by Russian biologist Aleksandr Oparin, the authors suggest that primordial organisms likely emerged from coacervates—organic molecule droplets that thrived under slow dynamic conditions. These insights ignite discussions surrounding evolutionary biology and the fundamental underpinnings of life itself.
An essential aspect of this research is the concept of chirality—the property that signifies an object cannot be superimposed on its mirror image. Within living organisms, homochirality refers to the dominance of a singular chirality in biological molecules, which plays a crucial role in molecular interactions. The research indicates a direct correlation between increased protein diffusion times and decreased stochastic fluctuations within the cell. This balance is vital for optimizing gene expression, underscoring the intricate relationship between protein dynamics and cellular functionality.
The implications of these findings extend beyond theoretical discussions and delve into the world of clinical medicine. The study accentuates the significance of liquid-liquid phase separation in the context of various diseases, particularly cancer. As articulated by co-author Marcos Minicucci, the ability of proteins associated with malignancies to compartmentalize can drastically influence their roles in cellular mutations and disease progression. The research also draws attention to the relevance of phase separation in ailments such as cataracts and neurodegenerative diseases, highlighting its potential therapeutic implications.
Moreover, recent studies have indicated that certain protein interactions, such as the phase separation linked with the protein FSP1, can be targeted for cancer treatment. The versatility of protein droplet formation in different diseases illustrates a complex narrative, where its effects can oscillate between being beneficial and detrimental, further complicating therapeutic approaches.
The research conducted by de Souza and his colleagues exemplifies the power of interdisciplinary collaboration in advancing scientific discovery. By blending principles from physics and biology, the team has constructed a novel framework for understanding cellular dynamics. The combined insights from physicists, biologists, and chemists underscore the importance of a multifaceted approach in tackling some of the most intricate problems in science today.
As the field continues to evolve, it will be exciting to observe how these concepts pave the way for new methodologies in both theoretical and practical applications, ultimately enhancing our comprehension of life at the molecular level and fostering advancements in medical science.