As the world grapples with climate change and the excessive greenhouse gases that contribute to it, the quest for sustainable energy sources is more urgent than ever. One promising avenue in this search is the ability to recycle carbon dioxide (CO2) into usable fuels. Researchers at the University of Michigan are pioneering a groundbreaking artificial photosynthesis system that effectively binds carbon atoms, producing ethylene—a critical hydrocarbon used widely in plastics. This innovative approach not only seeks to repurpose waste CO2 but also aims to minimize the reliance on fossil fuels, ushering in a new era of sustainable energy production.
Catalytic Efficiency of the University of Michigan’s System
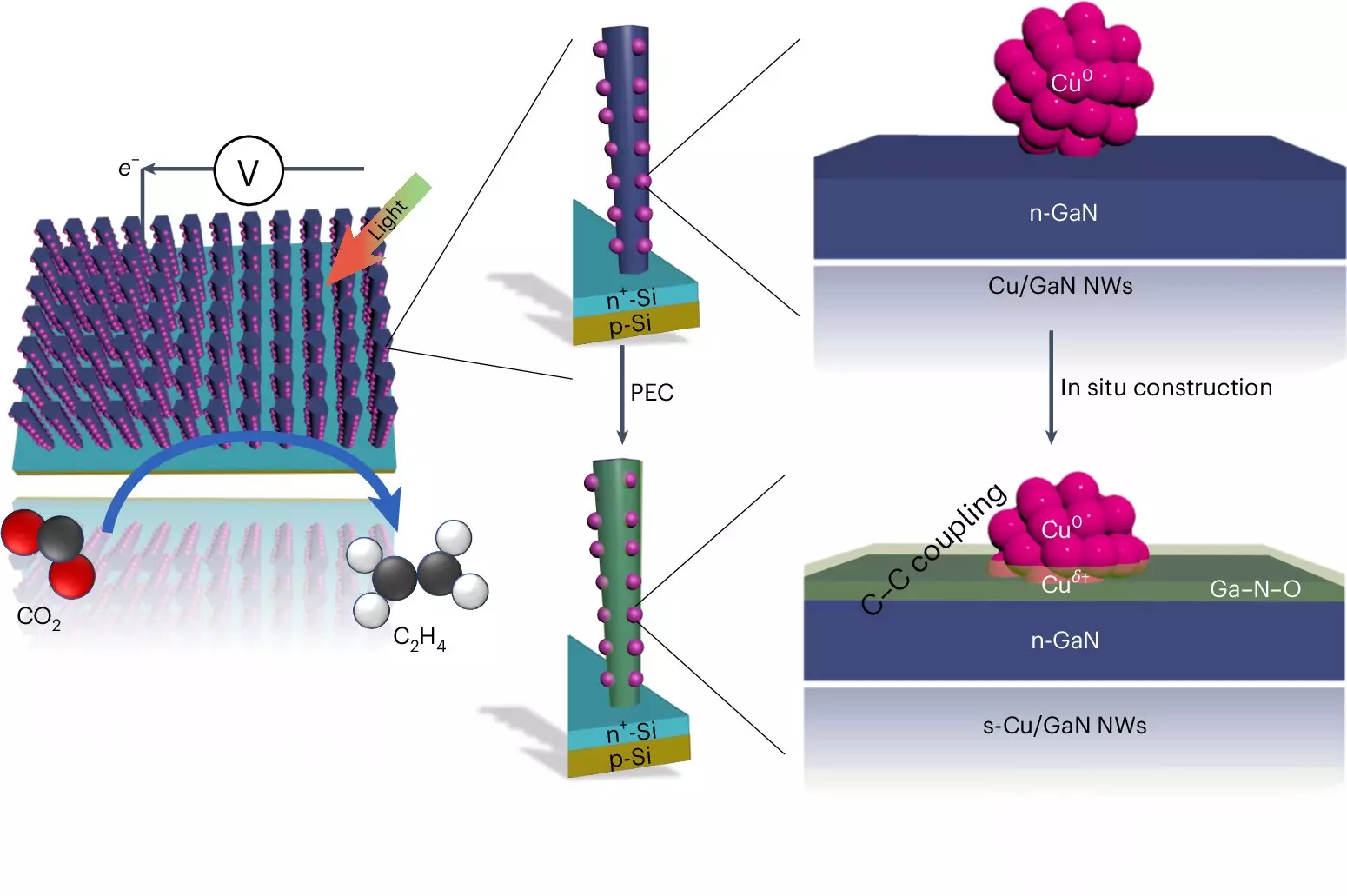
At the forefront of this technology is a sophisticated system that exhibits remarkable efficiency, yield, and longevity when converting CO2 into usable hydrocarbons. According to Professor Zetian Mi, whose team’s findings were published in *Nature Synthesis*, the performance of their device is five to six times superior to traditional solar energy-driven carbon dioxide reduction methods. Ethylene, being the most abundantly produced organic compound globally, is conventionally synthesized through energy-intensive processes involving fossil fuels. The Michigan team’s approach not only reduces the carbon footprint associated with ethylene production but also offers a sustainable alternative to the conventional methods that release additional CO2.
Central to the artificial photosynthesis system’s operation is the transformative process that utilizes two forms of semiconductors: gallium nitride nanowires and a silicon base. These nanowires, consisting of tiny structures just a few hundred atoms wide, play a critical role in absorbing sunlight. In essence, they harness solar energy to catalyze a series of reactions that split water molecules (H2O) into hydrogen and oxygen. This not only serves as a source of hydrogen for the ethylene production process but also contributes to the oxy-hydride semiconductor phenomenon, where the gallium nitride is converted into gallium nitride oxide.
Once the hydrogen is generated, it interacts with carbon sourced from CO2, facilitated by copper clusters located on the nanowires. This interplay of elements allows for the efficient conversion of hydrogen and carbon monoxide into ethylene—the product at the heart of this sustainable initiative.
One of the most significant achievements of the Michigan researchers is the device’s remarkable longevity. While many competing systems face rapid degradation, their artificial photosynthesis device operated for over 116 hours without performance decline, with some devices achieving up to 3,000 hours of continuous operation. This durability can, in part, be attributed to the synergistic relationship between gallium nitride and the water-splitting process, where the presence of oxygen enhances the catalytic activity and promotes a self-repairing mechanism.
Meanwhile, other catalysts, such as silver and copper, may show impressive initial efficiencies but become impractical due to their susceptibility to rapid wear and the limited operational timeline. The Michigan team’s findings unveil a paradigm shift in developing sustainable energy technologies with a focus on durability.
Future Directions: Beyond Ethylene
Looking ahead, the focus is not merely on generating ethylene but also on expanding the range of carbon-based compounds produced by this innovative system. Researchers aim to develop methods to synthesize longer-chain hydrocarbons, such as propanol and other liquid fuels. These advancements have the potential to revolutionize existing transportation technologies, paving the way for a more sustainable future. The ability to efficiently produce and transport liquid fuels could significantly alter the energy landscape, reducing dependency on fossil fuels while utilizing existing infrastructure.
The development of this advanced artificial photosynthesis system represents a crucial stride toward sustainability in energy production. By recycling CO2 into valuable hydrocarbons, the University of Michigan team not only addresses the pressing issue of greenhouse gas emissions but also innovates pathways for sustainable fuel production. As global energy demands grow, continuing to explore and enhance these technologies is essential. The insights gained from these research initiatives provide hope and direction in the quest for cleaner, more sustainable energy solutions that can satisfy the needs of modern society without compromising the health of our planet.