In the realm of science, photosynthesis stands as a critical process that supplies the energy driving life on Earth, harnessing sunlight to convert carbon dioxide and water into glucose and oxygen in plants and bacteria. Similarly, artificial mechanisms like photovoltaic systems emulate this process, converting light energy into electrical energy through the action of charged particles. Both phenomena hinge on a fundamental aspect of physics: the motion of electrons and charge transfer at the molecular level. Understanding these intricate processes offers potential advancements not just in natural biology, but also in synthetic designs like solar panels.
The mechanisms underlying charge transfer are rooted in ultrafast dynamics and quantum effects. When light engulfs a molecule, it induces a rapid redistribution of electronic density, a process that occurs on an incredibly short timescale, unveiling a world where electrons dance in synchrony with the nuclei. The ability to observe these dynamics with high temporal resolution elevates our understanding of the processes that not only occur in photosynthesis and photovoltaic technologies but also in various chemical reactions. Herein lies the challenge: capturing the first moments following photoionization—a phenomenon that remains elusive to researchers despite the plethora of advancements in ultrafast spectroscopic techniques.
Prominent among the tools available to researchers are ultrashort ultraviolet pulses sourced from high-order harmonics or free electron lasers. These innovative technologies enable scientists to manage and study the response of molecules during charge transfer while operating on demanding timescales, from femtoseconds (10^-15 seconds) to attoseconds (10^-18 seconds). However, the initial sequences of events following the prompt excitation of electrons remain inadequately described, creating a gap in the scientific literature that beckons for exploration.
In a groundbreaking study conducted by researchers from Politecnico di Milano and several partner institutions, including the Madrid Institute for Advanced Studies in Nanoscience (IMDEA Nanociencia) and the Autonomous University of Madrid, the veil of this mystery begins to lift. Published in *Nature Chemistry*, the findings showcase the power of attosecond extreme ultraviolet pulses in dissecting the ultrafast dynamics of molecular systems. This innovative study signifies a leap forward in understanding the electron-nucleus interactions in donor-acceptor molecules, which lie at the core of charge transfer phenomena.
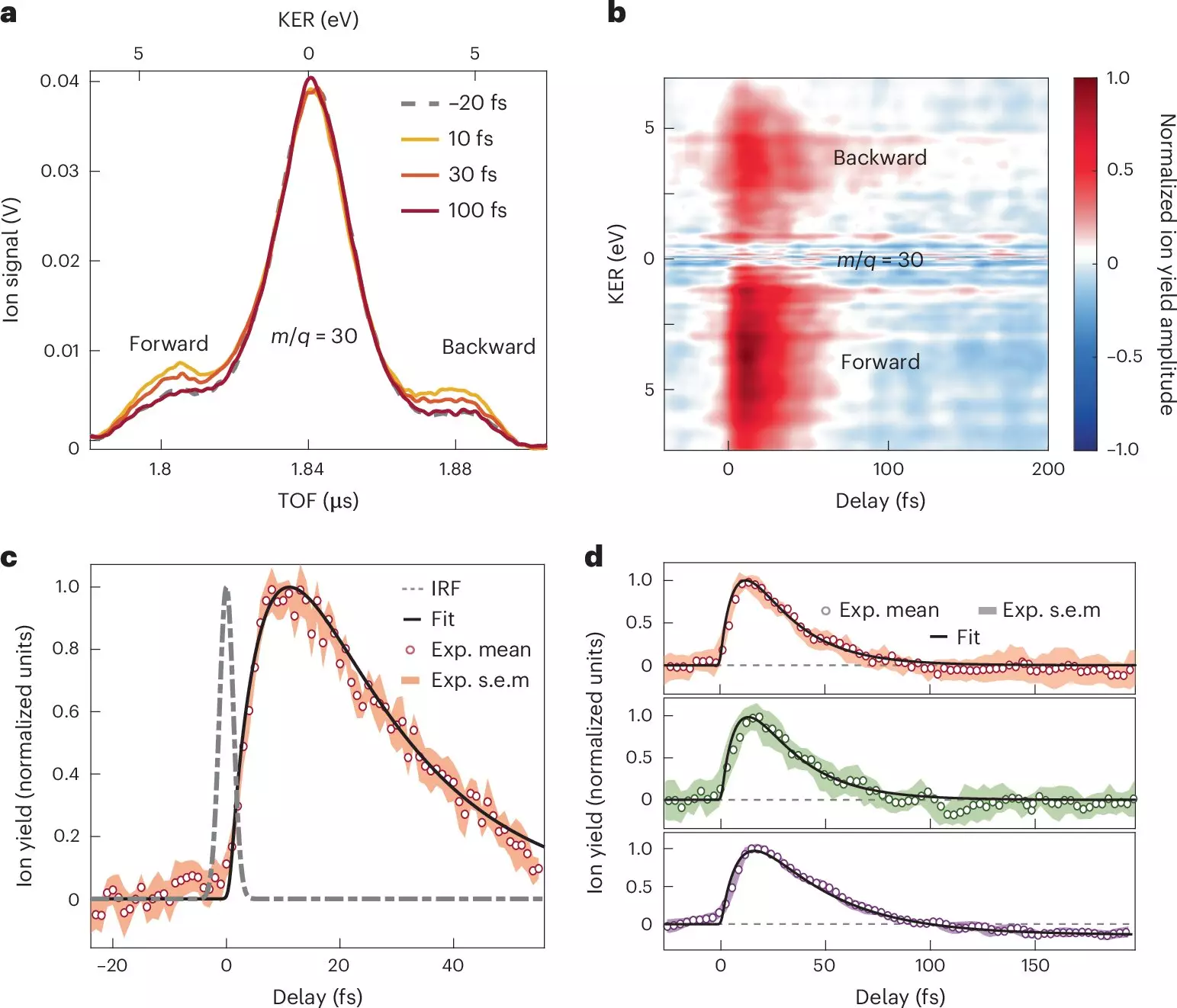
By strategically exposing nitroaniline molecules to attosecond pulse sequences, the research team successfully captured the initial fractions of time in which charge transfer occurs—an astonishing feat that contributes immensely to our theoretical understanding. Through the employment of techniques such as attosecond extreme-ultraviolet-pump and few-femtoseconds infrared-probe spectroscopy, coupled with sophisticated many-body quantum chemistry calculations, the nuances of these rapid molecular reactions began to surface.
The study revealed significant insights regarding the electron transfer from an amino group—a noted electron donor—that transpires in less than 10 femtoseconds. This rapid movement is not an isolated event; it is intricately connected with the synchronized motion of nuclei, reflecting a deep interplay between these subatomic particles. Following this swift transfer, the process continues with a nuclear relaxation phase that stretches over approximately 30 femtoseconds, as the excited electronic states of the molecular cation evolve.
Such findings illuminate the pivotal role of electron-nuclear coupling, shedding light on the broader implications for electron donor-acceptor systems in the context of photoionization. Furthermore, the research delineates the timescales necessary for charge migration across the molecular landscape, accentuating the corresponding structural changes that accompany this energetic dance. As the authors propose, these experimental results could refine our conventional understanding of electron transfer processes illustrated in educational diagrams, making them more reflective of the actual underlying dynamics.
This research not only opens doors to a deeper comprehension of molecular behavior and dynamics but also establishes a foundation for future investigations in attosecond science. As we continue to parse the complexities of molecular interactions, the insights gained from this work will likely contribute to enhancing the design of efficient energy-harvesting technologies and furthering our grasp of fundamental chemical processes. The intersection of quantum mechanics and molecular dynamics remains an exciting frontier, promising to enrich our understanding of both nature and engineered systems in remarkable ways.